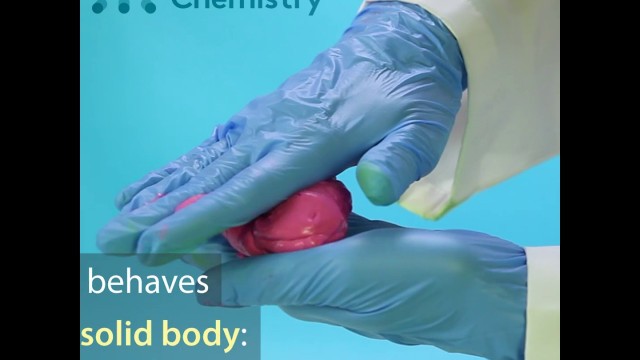

'For cool and safe experiments sign up to MEL Science here: https://goo.gl/9bgQIX Kids love to play with slime. It’s like a big piece of chewing gum, but you play with it with your hands. It’s like plasticine but it doesn’t stick to your hands, doesn’t leave marks on surfaces and doesn’t keep its form. In our experiment, we’ll show you how to make slime. Reagents and equipment: * PVA glue (30 g); * food coloring (2 g); * sodium tetraborate (5 g); * beaker; * distilled water. Step-by-step instructions Pour 30 g of PVA glue into a small beaker. Then add a small amount of food coloring, and mix until a uniform mass is formed. Add 10 ml of the sodium tetraborate solution and mix the resulting mass. In a few minutes the mass will thicken and turn into real slime! Processes description PVA glue is an emulsion of polyvinyl acetate in water, with a plasticizer and special additives. A solution of sodium tetraborate (Na₂B₄O₇•10Н₂O), also known as borax, is added to the PVA. When this mixture is stirred, the liquid gradually thickens and turns into a viscous slime, a soft rubber which you can form and stretch with your hands. The sodium tetraborate in the aqueous solution hydrolyzes, with the formation of an alkali and weak boric acid. And in the alkaline solution, the PVA transforms: it loses acetic acid and a polyvinyl alcohol is formed. The boric acid then connects chains of polyvinyl alcohol to each other. A cross-linked polymer is formed, which is much more viscous and less fluid than the initial polyvinyl acetate (PVA). The resulting slime has the properties of a non-Newtonian fluid. A non-Newtonian fluid is a fluid which sometimes behaves like a solid body and sometimes like a fluid. A non-Newtonian fluid can spread and flow, and can also be solid and bounce. The reason for this result is that these fluids are usually made from large polymeric molecules, which do not have very strong “adherence” to each other, so the molecules can slide over each other relatively freely. Safety precautions Don’t let children put the toy in their mouths. After playing with slime, you should wash your hands. Keep the slime in a clean jar with a lid, in a cold place.'
Tags: diy , slime , experiment , fun science experiments , cool experiments , easy science experiments for kids , easy Experiments , experiments to do at home , mel science , amazing experiment , cool science projects , science experiments at home , mel chemistry , fun experiments , chemistry lab , chemistry help , cool chemical reactions , at home science experiments
See also:

















comments